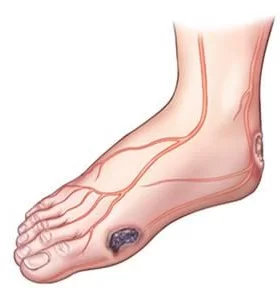
Amniotic stem cell rich treatments have been great for difficult wounds, such as in diabetics.
Amniotic membranes have been used extensively in plastic surgery for treating wounds that have been difficult to heal, as well as opthalmology for helping to heal corneal injury. Amniotic derived stem cell activator injections have been successfully used to promote spinal fusion during surgery, and as a scar barrier around the spinal cord.
Amniotic derived stem cell activator injections have been used over 10,000 times in the US with no reported adverse events, and hundreds of thousands of times in Europe and abroad. There are plenty of anecdotal reports regarding the use of amnion for treating tendinosis and tendinitis of the rotator cuff and elbow tendons. This includes both golfer’s elbow and tennis elbow. Results from these reports have been very promising.
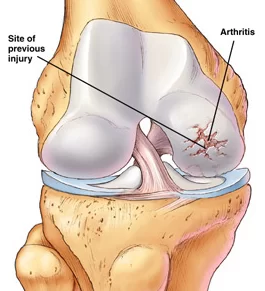
Additionally, anecdotal reports on the use of amnion for joint arthritis in both the spine and extremities has also been encouraging. Surgeons have started using the amnion to supplement surgical repair and reconstruction of ACL’s, Achilles tendons and rotator cuff tendons.
Amniotic derived stem cell activator injections are considered experimental by the FDA. None of the material on this website has been evaluated by the FDA, and no claims are being made. Outcomes vary between individuals.