Worldwide, over 528 million individuals suffer with osteoarthritis pain, with the knee being the most common affected joint. The amount of people affected has increased by over 100% within the past twenty years and will increase by another 100% over the next twenty!
Interestingly, women (60%) are more commonly affected than men, with the majority of those affected being over age 55 (70%). Osteoarthritis can greatly reduce one’s quality of life. It makes movement painful and difficult, which can stop people from participating in home, work or social activities. This can lead to mental health impacts, trouble sleeping and problems in relationships.
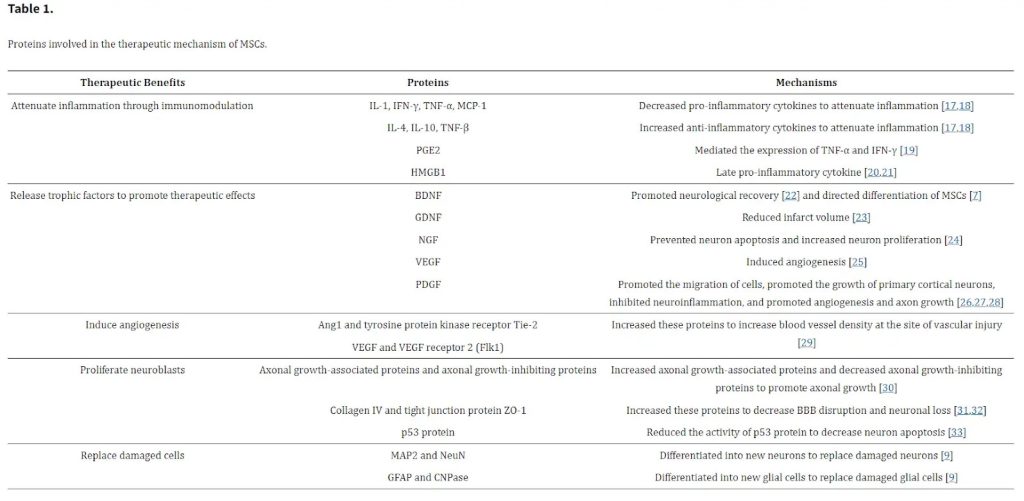
Guide to Stem Cell and Exosome Therapy for Osteoarthritis What happens during osteoarthritis development? Osteoarthritis (OA) refers to a common chronic degenerative joint disease, namely the degenerative injury of articular cartilage caused by multiple factors (e.g., aging, obesity, fatigue injury, trauma, joint congenital abnormalities, joint deformity, etc).
Pathological changes largely include articular cartilage destruction, subchondral osteosclerosis and synovial hyperplasia. OA occurs primarily after middle age, and it is more widespread in women than in men.
Age related ’wear and tear’, chondrocytes’ poor response to growth factors, altered biomechanical properties of articular cartilage, mitochondrial dysfunction, oxidative stress and inflammation are all implicated in the pathogenesis of OA, highlighting the multifactorial and complex nature of this degenerative joint disease.
Eventual decreases in the number of chondrocytes with age results in impaired production of extracellular matrix proteins.
Our doctors at R3 Stem Cell clinic are equipped to address these challenges with advanced stem cell therapies.