Wang et al published a meta-analysis in 2021 where they evaluated 18 human studies on IBD, including 360 patients. In the studies that looked at remission rates with mesenchymal stem cells, rates at 1, 3, 5, 12, 24, and 36 months after stem cell therapy were 43%, 68%, 73%, 54%, 52%, and 46%, respectively, thus both high and stable.
In 2006, Onken et al published an abstract on mesenchymal stem cells used to treat 10 patients with active Crohn’s Disease (CDAI >220, C-reactive protein ≥ 5 mg/L) refractory to treatment with corticosteroids, immune modulators and infliximab.
The patients were randomized to two groups, both of which received two intravenous doses of MSCs, spaced one week apart. One group received high-dose MSCs (8 million MSCs/kg), while the other group received low dose MSCs (2 million MSCs/kg). The primary endpoint was percentage clinical response, defined as a reduction in the CDAI score of ≥ 100 points.
All subjects presented a mean reduction in CDAI score of 105 points on day 28. The CDAI scores decreased to a greater extent in the high-dose group.
In 2014, Forbes et al published a study looking at donor mesenchymal stem cells in patients with luminal Crohn’s Disease who were nonresponders to traditional therapies. Sixteen patients were given intravenous infusions of allogeneic MSCs (2 million stem cells/kg body weight) weekly for 4 weeks.
Among the 15 patients who completed the study, the mean CDAI score was reduced from 370 to 203 at day 42 (P < .0001). That’s a decrease of 46%!
The mean CDAI scores decreased after each MSC infusion (370 before administration, 269 on day 7, 240 on day 14, 209 on day 21, 182 on day 28, and 203 on day 42). Twelve patients had a clinical response, 8 had clinical remission.
In 2018, Zhang et al published the BEST study to date on umbilical cord MSC’s for Crohn’s Disease. The study sought to investigate the efficacy and safety of UC-MSCs for the treatment of Crohn’s Disease.
Eighty-two patients who had been diagnosed with CD and had received steroid maintenance therapy for more than 6 months were included in this study. Forty-one patients were randomly selected to receive a total of four peripheral intravenous infusions of 1 million umbilical cord stem cells/kg, with one infusion per week. Patients were followed up for 12 months.
The Crohn’s disease activity index (CDAI), HarveyBradshaw index (HBI), and corticosteroid dosage were assessed. Twelve months after treatment, the CDAI, HBI, and corticosteroid dosage had decreased by 62.5, 3.4, and 4.2 mg/day, respectively, in the UC-MSC group.
In the control group, the CDAI, HBI, and corticosteroid dosage had decreased by 23.6, 1.2 and 1.2mg/day, respectively.
The superiority of umbilical cord mesenchymal stem cells was statistically significant, and patients were able to avoid the side effects of chronic steroid use! No serious adverse events were observed. The conclusion was that umbilical cord mesenchymal stem cells were effective in the treatment of Crohn’s Disease and produced mild side effects.
Below you will see before and after endoscopic results of two patients in the study who received the mesenchymal stem cells. Complete colon healing!
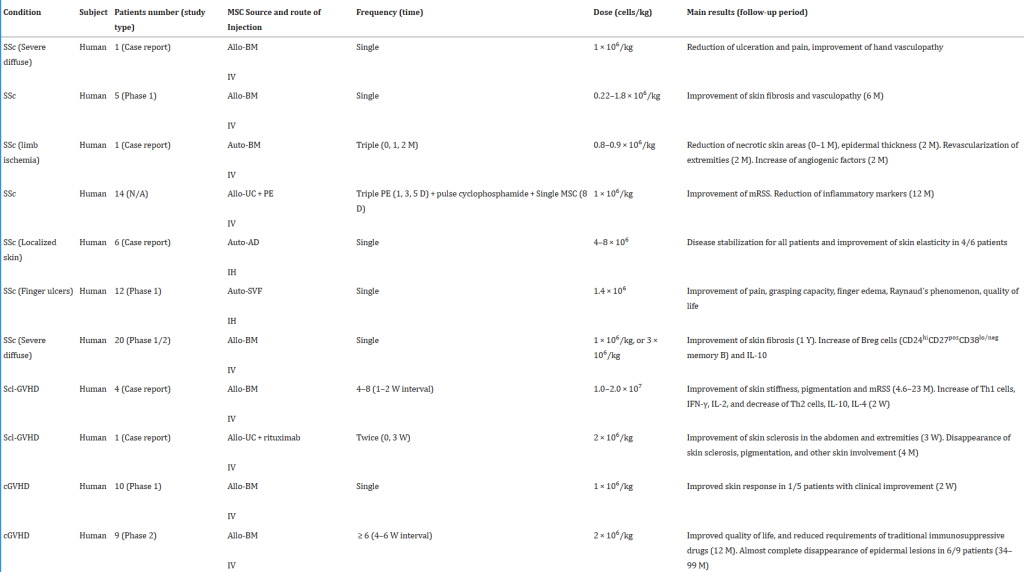
Below is a table of several clinical trials showing the dose of stem cells used, number of patients, follow up duration and the outcome. The results are extremely impressive!