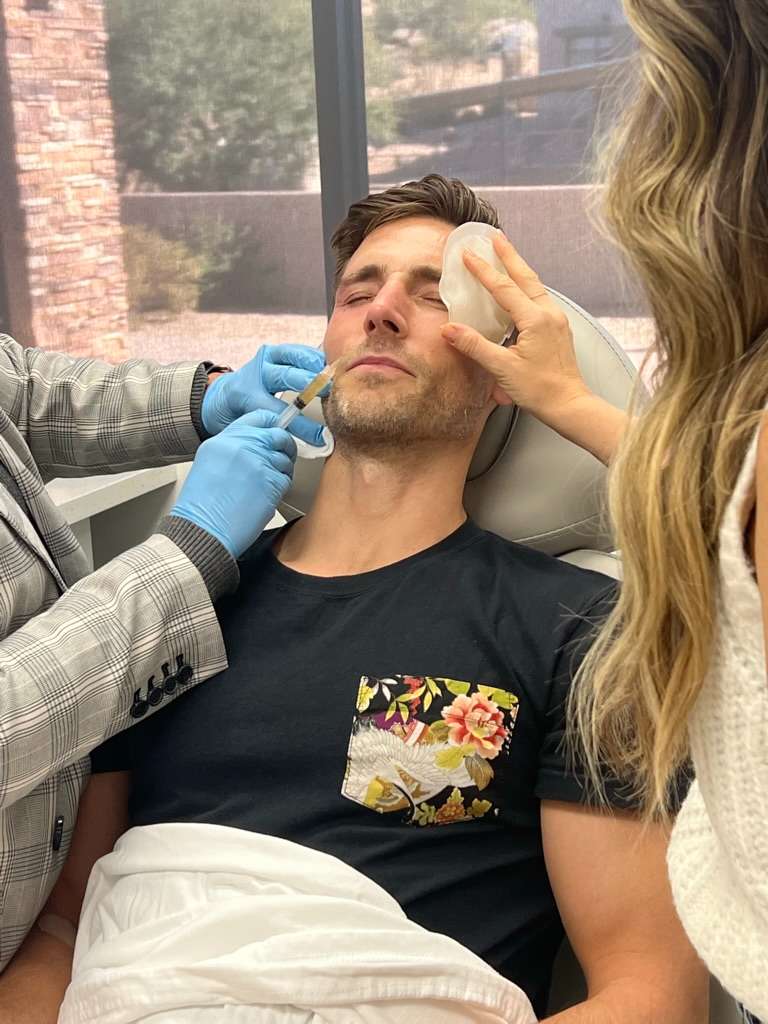
The primary treatment outcome to be measured was change in pain intensity and the secondary outcome measure was any reduction in the daily consumption of anti-neuropathic medication. Subjects were reviewed for safety evaluation for any clinical sign of trigeminal nerve deficit (paresthesia, dysesthesia), facial nerve paresis, infection, and unusual swellings or lesions at the injection sites.
The results showed a reduction in pain intensity scores from the stem cell treatment in 7/9 patients (one patient was lost to follow-up). Five of the most positive responders also reduced their need for gabapentin medication. The majority of subjects, however, reported a positive effect from stem cell therapy with pain reduction in addition to lowering anti-neuropathic medication dose to enable an improved quality of life with fewer drug side effects.
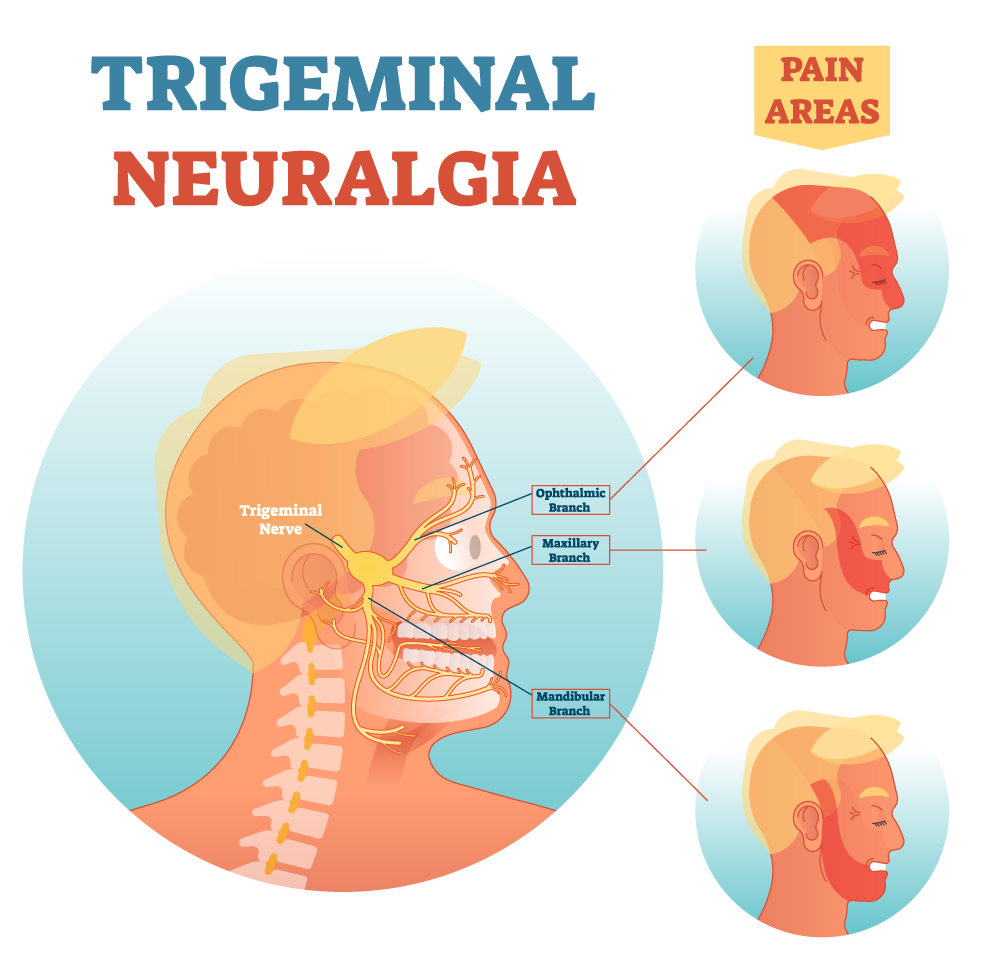
There were no visible deleterious changes at any of the injection sites. In addition, there were no changes to normal nerve physiology of the involved cranial nerves where stem cells were administered; specifically, no numb¬ness, tingling, dysesthesia on the lower lip, chin, lateral border of the tongue or face (trigeminal nerve branches V2 and V3), and no report of motor nerve dysfunction to the face (C7, facial nerve) or jaw (motor branch of the trigeminal nerve). In addition, no infection of the environment was observed where the stem cells were injected.