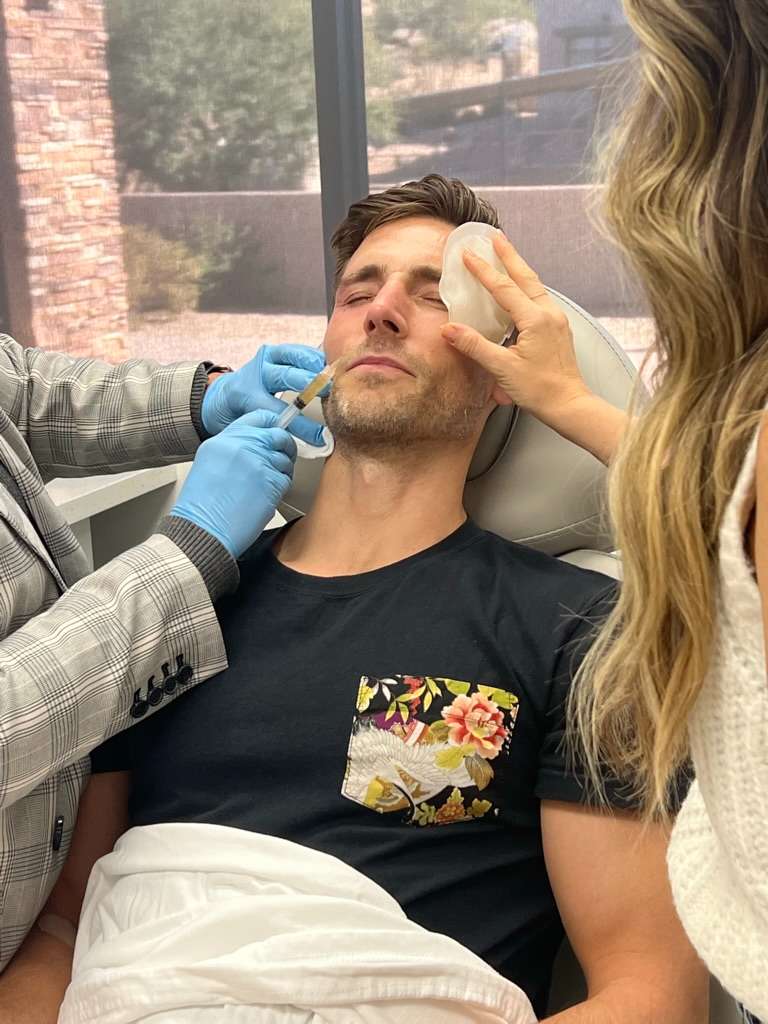
Because stem cell therapy for stroke does benefit from repeat treatments, it’s important to make it affordable. Repeat therapies can help people achieve additional speech, function and mobility improvements. So, a lot of patients seek additional treatments at R3 Stem Cell every six to twelve months.
Unfortunately, stem cell clinics in Colombia, China and Panama charge over $20,000 USD for stroke treatment. Because the one treatment costs so much, how are individuals supposed to budget for that every year?? R3 Stem Cell’s fees are less than half that for 100 million high-quality stem cells!
 For the past decade, R3 Stem Cell’s Centers globally have performed over 23,000 regenerative procedures in six countries. Several hundred have been for either ischemic or hemorrhagic strokes. Patient satisfaction across all conditions treated is 85%!
For the past decade, R3 Stem Cell’s Centers globally have performed over 23,000 regenerative procedures in six countries. Several hundred have been for either ischemic or hemorrhagic strokes. Patient satisfaction across all conditions treated is 85%!
R3 combines safety, effectiveness and affordability for the therapies. Internationally, the Intellicell is used, which is culturing the most active mesenchymal stem cells to create the “smartest” stem cell in the world!
Our experience with stroke patients has been extensive, and our Success Stories on R3’s YouTube Channel are impressive. You can visit the channel Success Story Playlist HERE. R3 Stem Cell offers free consultations for individuals to discuss whether regenerative therapy is indicated for their stroke recovery. Simply call +1 (844) GET-STEM or +1 (480) 808-7057 to schedule yours!


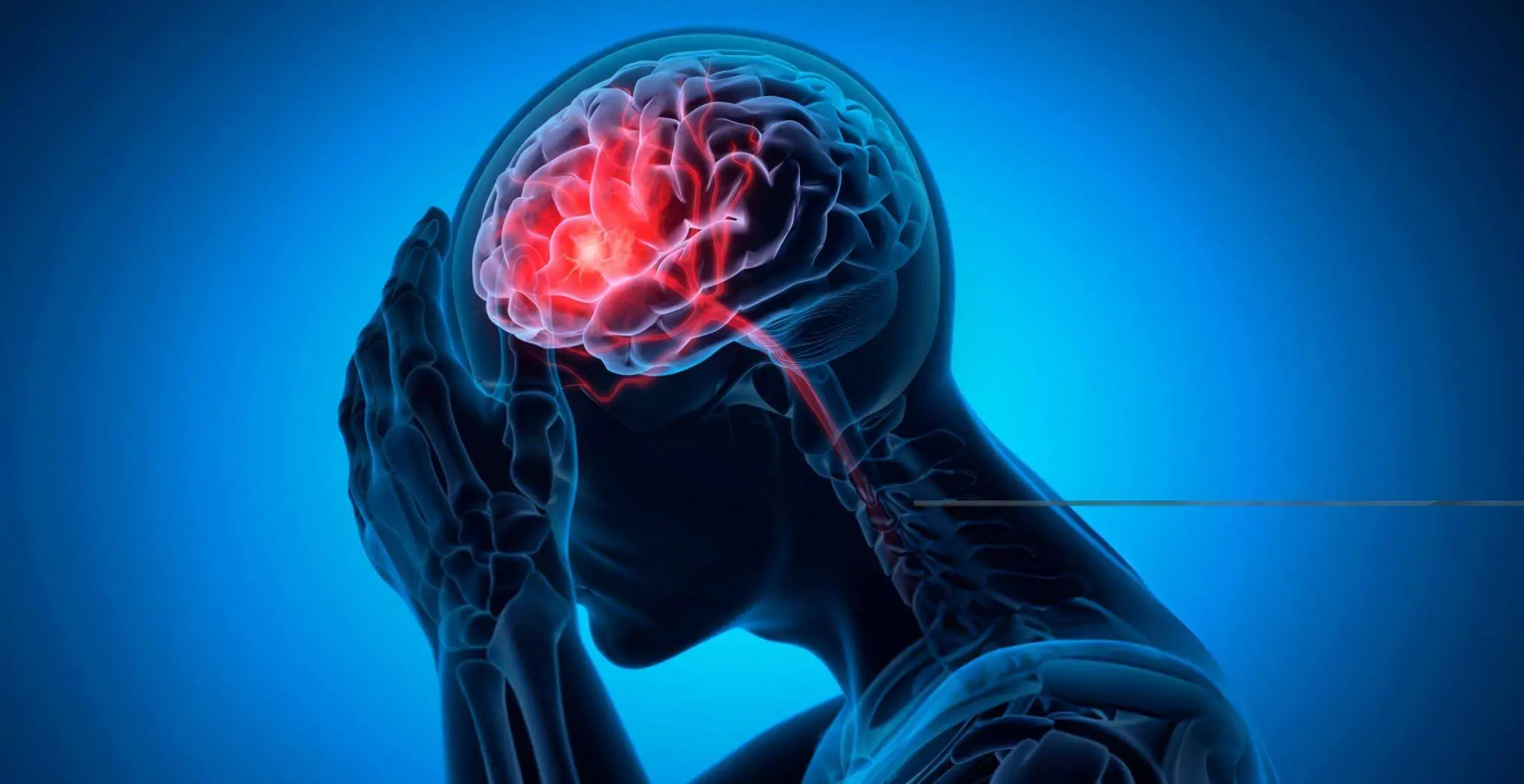





 For the past decade, R3 Stem Cell’s Centers globally have performed over 23,000 regenerative procedures in six countries. Several hundred have been for either ischemic or hemorrhagic strokes. Patient satisfaction across all conditions treated is 85%!
For the past decade, R3 Stem Cell’s Centers globally have performed over 23,000 regenerative procedures in six countries. Several hundred have been for either ischemic or hemorrhagic strokes. Patient satisfaction across all conditions treated is 85%!