A 2014 study in the Journal of Translational Medicine evaluated umbilical cord mesenchymal stem cells for transplantation and compared neurofunctional outcomes of patients suffering sequelae of thoracolumbar spinal cord injury that were treated with stem cell transplantation, rehabilitation training, or no treatment.
A 2014 study in the Journal of Translational Medicine evaluated umbilical cord mesenchymal stem cells for transplantation and compared neurofunctional outcomes of patients suffering sequelae of thoracolumbar spinal cord injury that were treated with stem cell transplantation, rehabilitation training, or no treatment.
34 cases of thoracolumbar spinal cord injury that were graded ‘A’ by the AIS grading system were randomly divided into 3 groups: the stem cell transplantation group, the rehabilitation therapy group, and the blank control group. The stem cell group received 40 million umbilical cord mesenchymal stem cells through intrathecal application on two separate procedures, 10 days apart.
Through scaled ratings and urodynamic examinations, this study proved that transplantation of UCMSCs has advantages in neurofunctional recovery in comparison with rehabilitation therapy and self-healing alone. The stem cell transplantation group improved significantly in motor function (P = 0.012); that is, the muscle strength of the waist, abdomen, and lower limbs increased. Seven of the 10 patients had their muscle strength increased from level 0 to level 1 or 2 (data not shown), and motor function of the paralyzed limbs improved as muscle strength increased. The rehabilitation group and blank control group also showed some improvements but the difference was not statistically significant.
Regarding muscle tension, excessive muscle tension significantly decreased (P = 0.007) after stem cell transplantation. Eight patients showed excessive muscle tension before treatment, and 7 of them (87.5%) had their muscle tension decreased (data not shown); the rehabilitation group and blank control group showed no significant improvements in muscle tension (P > 0.05).
As to self-care ability, the stem cell transplantation group significantly improved in activities such as bed-chair transfer, bowel and urinary retention, and ground movements, which are related to a decrease in excessive muscle tension and an improvement in movement ability of the paralyzed limbs. The rehabilitation group showed some improvements but the differences were not statistically significant (P > 0.05). The self-care ability of the blank control group decreased (P > 0.05), most likely representing functional decline of the limbs due to lack of treatment and use.
This study demonstrated that umbilical cord mesenchymal stem cell transplantation is effective in the treatment for sequelae of thoracolumbar spinal cord injury. This method can alleviate lower limb muscle tension, increase limb strength, and improve urinating function. The method’s efficacy is more significant in comparison with rehabilitation therapy, and no adverse effects were found.



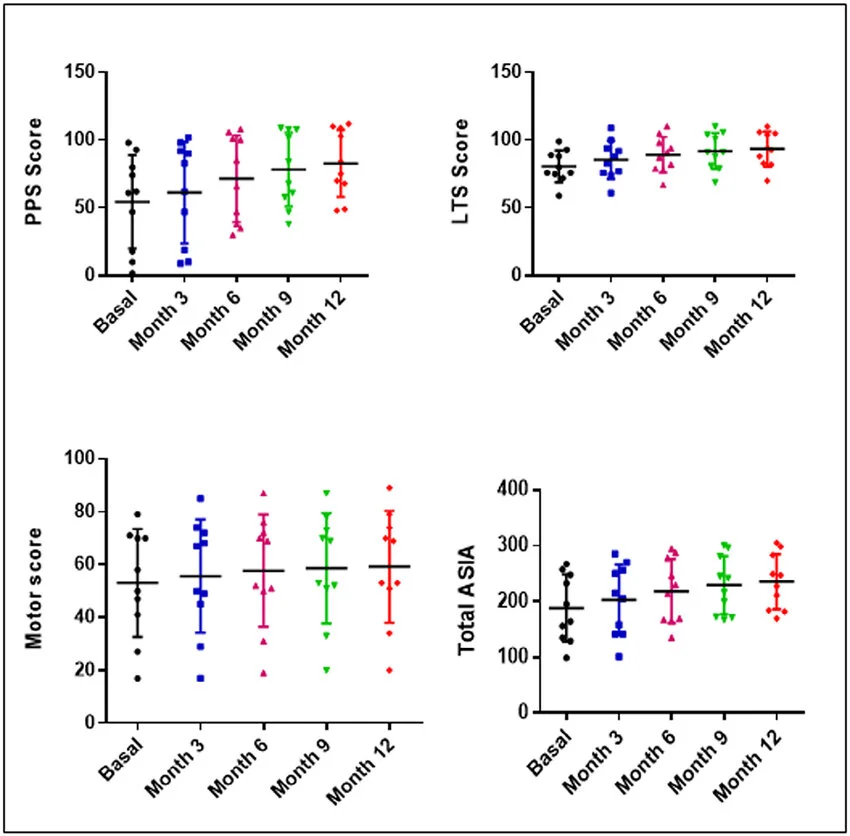 A 2017 study out of Spain evaluated ten patients with established incomplete SCI receiving four subarachnoid administrations of 30 million autologous bone marrow MSCs, supported in autologous plasma, at months 1, 4, 7, and 10 of the study, and were followed until the month 12. Urodynamic, neurophysiological, and neuroimaging studies were performed at months 6 and 12, and compared with basal studies.
A 2017 study out of Spain evaluated ten patients with established incomplete SCI receiving four subarachnoid administrations of 30 million autologous bone marrow MSCs, supported in autologous plasma, at months 1, 4, 7, and 10 of the study, and were followed until the month 12. Urodynamic, neurophysiological, and neuroimaging studies were performed at months 6 and 12, and compared with basal studies.