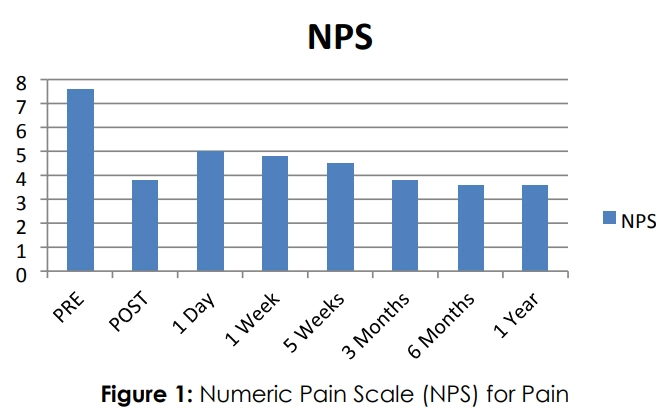
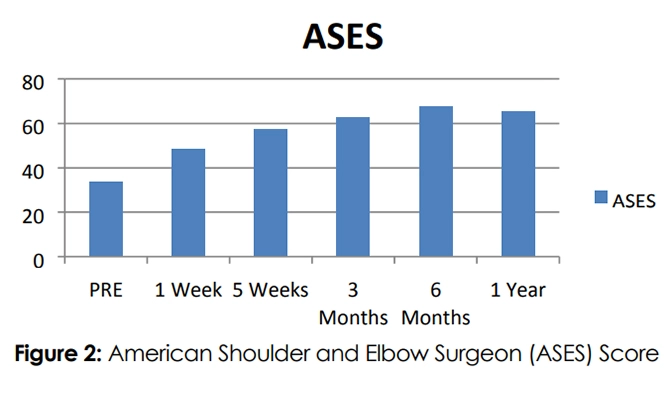
Similar to the research mentioned above, R3 Stem Cell's outcomes for rotator cuff disease have been exceptional! The patient satisfaction rate is 85% year over year. Patients typically experience pain reduction along with improved shoulder function. Keep in mind results cannot be guaranteed and will vary between individuals.
It may take a couple months to see all the improvements, as it can take that long to build up new blood flow. It should be noted, again, that stem cell therapy is not a cure, and may need to be repeated every few years or so for continued benefit.
Because stem cell therapy for rotator cuff disease is not a permanent cure, it's important to make it affordable. Repeat therapies can help maintenance and/or achieve additional improvements for musculoskeletal health. So a lot of patients seek additional treatments at R3 Stem Cell every few years.
R3 Stem Cell's fees are less than half what comparable (and reputable) regenerative clinics charge. Be wary of clinics trying to pass off PRP as a stem cell therapy. If they mention only taking your blood for the treatment, it is NOT a stem cell treatment!