 neurotrophic factor, and traditional Chinese medicines. However, their conditions were still progressively aggravated.
neurotrophic factor, and traditional Chinese medicines. However, their conditions were still progressively aggravated.
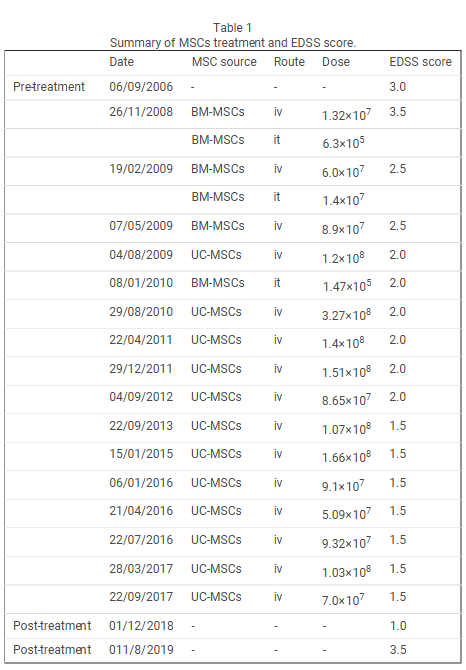
In this study, UCMSC transplantation was used to treat two patients with multiple sclerosis for a total of seven times of treatments. No obvious adverse reactions or residual pathological syndromes appeared during transplantation. The physiological examination and MRI results revealed normal indexes. Toxic reactions of the UCMSCs were not detected during the 8-year follow-up. Clinical signs and symptoms were mitigated in the two patients after transplantation.
The onset frequency was compared within the same time after transplantation, and the average annual onset frequency in the transplant patients were remarkably less than before transplantation. The EDSS scores demonstrated that the clinical symptoms were mitigated in Patient 1. At the time of this writing, his symptom was stable and not progressive.
After the first and second transplantations, the symptoms of Patient 2 were progressive. Therefore, we shortened the time interval and administered cell therapy. His condition was stable at the time of this writing. The MRI findings showed that the number of foci was obviously reduced after transplantation, suggesting that UCMSC transplantation promoted remyelination.
The researchers wrote, “In summary, UCMSCs play an important role in immune regulation and neural protection. UCMSCs can regulate pathological immune responses and antibody attacks in the body. The neuroprotective effect is strongly associated with the mechanism of promoting remyelination. Our findings confirm that UCMSCs have functions of immune regulation and nerve protection, indicating the feasibility of UCMSC transplantation for multiple sclerosis.
A newly published study (2024) titled “Human Umbilical Cord–Derived Mesenchymal Stem Cells in the Treatment of Multiple Sclerosis Patients: Phase I/ II Dose-Finding Clinical Study” looked at umbilical cord mesenchymal stem cell therapy in two different dosings.
Patients were divided into two groups: Group A comprised 20 MS patients who received two doses, each with a total of 150 million UC-MSCs divided into two injections, 50 million administered through the IV route, and 100 million administered through the intrathecal (IT) route. A month later, another similar dose was administered. At 3 months, 8 to 10 ml of UC-MSCs Conditioned Media was delivered IT.
On the other hand, Group B comprised 15 MS patients who received one dose of 150 million UC-MSCs divided into 50 million administered through the IV route and 100 million administered through IT route. At 3 months, 8 to 10 ml UC-MSCs Conditioned Media was delivered IT. Prior to each IT treatment, the same volume injected was withdrawn as CSF to maintain the CNS pressure and decrease the potential post-IT injection headache.
So Group A received TWO treatments (a month apart) and Group B received just one. Both received conditioned media IT at 3 months.
Although the intravenous (IV) administration of stem cells is the most common, a combined route of UC-MSCs injection could be an important efficacy element, as tracking studies have shown that MSCs injected intrathecally (IT) migrate to the site of injury in the white matter of the brain. The migration of allogenic MSCs injected IT was found to be through the cerebrospinal fluid (CSF), reaching different locations of the CNS where they would persist without the need for immune suppressants.
The results indicated that both stem cell treatment protocols halted the overall progressive deterioration in the EDSS during the 12-month follow-up period. An improvement at all time points for both groups, except for the 3 months for Group A, was observed. The walking and balance results are in line with the EDSS outcomes in Group B, while a gradual deterioration was noted in Group A.
In summary, this study demonstrated the safety and efficacy of both treatment protocols with comprehensive assessment tools, using an allogenic stem cell source originating from a single umbilical cord donor and expanded in vitro. The various aspects studied point to halting and reversing MS symptoms using stem cell therapy with parallel effects on the cellular and gene expression levels.
There is an advantage of administering two doses compared to one, which warrants more extensive studies on larger numbers of MS patients. Examining the addition of more doses and a more extended followup period is recommended for future studies.
The findings of this study emphasize the critical role of regenerative medicine in managing MS. Optimizing the dose of treatment is an essential milestone for the standardization of protocols to attain a safe cellular treatment of MS symptoms. The many aspects studied detected a reversal of some MS symptoms and stabilization of others. The clear advantage of administering two doses of UC-MSCs instead of one in this study, in addition to one dose of CM, is encouraging.
Of the several types of MSCs, UCMSCs are the best option for MS treatment for several reasons. These cells can do a faster self renewal than other MSCs, can differentiate into three germ layers, and can accumulate in damaged tissue or inflamed areas. They also have their own advantages that makes them the choice of MS therapy. First, the separation of the cells from the UC is easy, painless, and without ethical issues. Second, the amount of stem cells produced per unit area is high. Third, the cost of stem cell transfusion from the UC is not expensive.
Fourth, these cells are very safe to use. Based on the studies presented in the section about UCMSCs, these cells would be considered as a safe and alternative option for treatment of the neurological parameters of MS, through results confirmed by EDSS, the ninehole peg test, the expanded EDSS rating neurologic impairment, and the 25-foot walking time.