Call to Schedule Free Consultation at Over 45 Centers Worldwide!
- Home
- SUCCESS STORIES
- About
- Stem Cells
- Conditions
Autoimmune
Cardiovascular
Endocrine
Gastrointestinal
Liver
Musculoskeletal
Neurological
Other
Respiratory/Pulmonary
Reproductive
Renal/Kidney
Urologic
- Videos
- Locations
- Home
- SUCCESS STORIES
- About
- Stem Cells
- Conditions
Autoimmune
Cardiovascular
Endocrine
Gastrointestinal
Liver
Musculoskeletal
Neurological
Other
Respiratory/Pulmonary
Reproductive
Renal/Kidney
Urologic
- Videos
- Locations



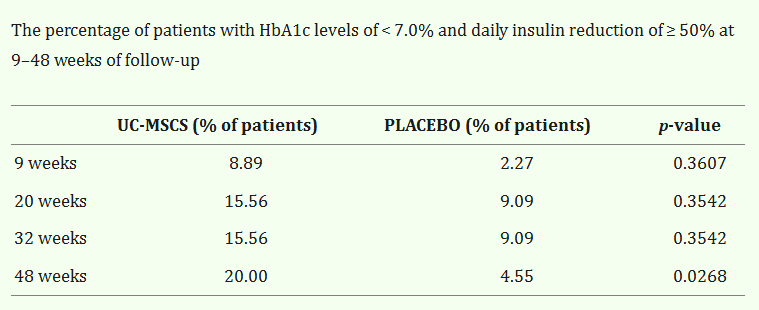 In a meta-analysis titled, “Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus “, 22 eligible clinical trials reporting stem cell-based therapy for DM with a total of 524 patients were evaluated. Both Type 1 and Type 2 DM were evaluated.
In a meta-analysis titled, “Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus “, 22 eligible clinical trials reporting stem cell-based therapy for DM with a total of 524 patients were evaluated. Both Type 1 and Type 2 DM were evaluated.