Call to Schedule Free Consultation at Over 80 Centers Worldwide!
Call to Schedule Free Consultation at Over 80 Centers Worldwide!
Advanced CP Treatment
R3 Stem Cell offers advanced solutions for those living with cerebral palsy.
Free Download: Stem Cell Therapy for Cerebral Palsy (CP)
Disclaimer: The information provided by R3 Stem Cell is for educational purposes and is not a substitute for professional medical advice, diagnosis, or treatment. Individual results may vary and are not guaranteed. The FDA considers stem cell therapy experimental at this point.
Any claims made on this website refer to procedures performed OUTSIDE of the USA. R3 Stem Cell has clinics in Mexico, Philippines, South Africa, Turkey, India, Pakistan.
Every day, R3 Stem Cell Treatment Center receives inquiries worldwide from individuals asking if stem cell therapy can help with Cerebral Palsy. Spoiler alert: It can help a lot! In this guide, we’ll go through the basics of how stem cells and exosomes work, the latest research, and what to expect with a regenerative procedure.
Cerebral palsy (CP), the most prevalent motor disorder of childhood, affects two to three per 1,000 live births. CP typically results from in utero or perinatal brain injuries such as hypoxic insult, hemorrhage, or stroke.
Affected children have varying degrees of functional impairments from mild limitations in advanced motor skills to severely limited self-mobility despite the use of assistive technology, often resulting in a lifelong inability to function independently.


Despite extensive treatment, neurological impairments still eventually lead CP patients to lifelong disability. While traditional treatment can bring improvement for sufferers of mild to moderate CP, severe CP lacks effective intervention options.
To date, no disease-modifying treatments have been found in CP. Current therapies focus on treating disabilities and managing associated comorbidities. The integral treatments include physical therapy and rehabilitation, but they have limited effectiveness in most cases.
In recent years, stem cell transplantation has emerged as a promising treatment strategy in clinical practices and various clinical trials. Studies on stem cell treatment for cerebral palsy provide a new treatment strategy and have shown exceptional results.
Compared to other types of stem cells, human mesenchymal stem cells (hMSCs) offer potential advantages of easy accessibility, immunosuppression, and low immunogenicity, making them attractive and promising in treating various diseases.
A meta-analysis published in 2020 evaluated four studies totaling 189 participants. A meta-analysis pools results from multiple studies to potentially achieve statistical significance.
GMFM scores (Gross Motor Function Measure) are crucial for evaluating changes in gross motor function for CP after interventions. They help determine the effectiveness and benefit of interventional therapy by measuring changes in gross motor skill acquisition in children with CP.
Children’s gross motor function is commonly evaluated by rehabilitation specialists using GMFM scores. The GMFM scored items consist of 5 parts:
Ready to explore innovative treatments for cerebral palsy? Contact our specialists at R3 Stem Cell Therapy Clinic at (844) GET-STEM to learn how our advanced stem cell treatment can help improve motor function and quality of life.
The items are scored on a four-point scale:
Higher GMFM scores indicate better capacity and favorable prognosis in children with CP.
To provide reliable and high-quality evidence, the meta-analysis authors included three randomized controlled trials of mesenchymal stem cell (MSC) therapy in CP. The pooled results showed that MSC therapy significantly increased GMFM scores in children with CP compared to the control group.
Moreover, a subgroup analysis of GMFM scores at 3, 6, and 12 months showed that MSC therapy significantly increased GMFM scores at all three time points.
Discover how R3 Stem Cell’s advanced treatments at our center can enhance functional outcomes for cerebral palsy patients. Call (844) GET-STEM for a personalized consultation today with our experienced doctors.
CFA is primarily used to evaluate functional improvement and therapeutic effects in patients with cerebral palsy (CP). A pooled analysis indicated that mesenchymal stem cell (MSC) therapy significantly improved CFA scores compared to the control group.
Researchers conducted a subgroup analysis on CFA scores, which revealed:
These results were observed in children with CP compared to the control group.
The study concluded that MSC therapy for CP improved the comprehensive function of patients with high-quality evidence.
Explore innovative CP treatments with R3 Stem Cell. Call (844) GET-STEM for a free consultation on our advanced stem cell therapies.
Every day, R3 Stem Cell Treatment Center receives inquiries worldwide from individuals asking if stem cell therapy can help with Cerebral Palsy. Spoiler alert: It can help a lot! In this guide, we’ll go through the basics of how stem cells and exosomes work, the latest research, and what to expect with a regenerative procedure.
Excessive salivation is a common symptom in CP patients, significantly affecting their health status. Umbilical cord mesenchymal stem cell (UCMSC) transplantation was found to significantly improve drooling severity and frequency.
No significant differences were observed in other adverse events such as fever, vomiting, anorexia, and urticaria. Importantly, no serious adverse events were reported in the included studies.

Manage CP symptoms effectively with R3 Stem Cell. Call (844) GET-STEM to explore our innovative therapies.
Every day, R3 Stem Cell Treatment Center receives inquiries worldwide from individuals asking if stem cell therapy can help with Cerebral Palsy. Spoiler alert: It can help a lot! In this guide, we’ll go through the basics of how stem cells and exosomes work, the latest research, and what to expect with a regenerative procedure.

A randomized, placebo-controlled trial in China involved 54 CP patients:
The hUCB-MSC infusion group showed significantly higher improvements in:
These improvements were observed at 3, 6, 12, and 24 months post-treatment, with particularly significant improvement in gross movement.
Duke University conducted a phase II, randomized, double-blind, placebo-controlled, crossover study on autologous cord blood (ACB) infusion in children aged 1 to 6 years with CP.
Stay informed about CP treatment breakthroughs. Contact R3 Stem Cell at (844) GET-STEM for the latest research-backed therapies.
The observation that higher cell doses led to better responses is crucial for future studies. While the initial target was 1-5 × 10^7 total nucleated cells/kg based on safety data from allogeneic cord blood transplantation, additional analyses revealed significant improvements in gross motor function when ACB was administered at doses higher than the mean.
Duke University conducted a follow-up study using allogeneic cord blood for CP in 91 children aged 2 to 5 years. The study compared three groups:

A 2015 safety study by Feng et al investigated the use of allogeneic umbilical cord blood stem cells in patients with severe cerebral palsy (CP). The study, conducted in China, involved 47 patients who received 20 to 30 million cells per injection, with 4-8 injections administered based on individual health conditions.
Key findings:
Duke University conducted a follow-up study using allogeneic cord blood for CP in 91 children aged 2 to 5 years. The study compared three groups:
Significant observations:

A 2020 study by Gu et al explored the use of human umbilical cord mesenchymal stem cells (hUC-MSC) for CP treatment. The study involved 39 patients randomized into treatment and control groups.
Results:
A 2021 study by Amanat et al evaluated the safety and efficacy of intrathecal administration of umbilical cord mesenchymal stem cells in children with CP.
Key findings:
Experience safe, effective CP treatments. Schedule your consultation with R3 Stem Cell at (844) GET-STEM today.
A 2015 Russian study investigated the effects of stem cell therapy on 80 pediatric patients with cerebral palsy (CP). The average age of participants was 12 years, and all had a clinically confirmed CP diagnosis. The study included:

Many patients had additional conditions, including:
Notably, 55 children exhibited delays in physical and mental development.
Each treatment dose averaged 250 million total nucleated cells per infusion. The cell administration was well-tolerated, with no acute or delayed adverse reactions reported, such as allergic reactions, headache, waist pain, fever, or vomiting.
The results were highly encouraging:
The study revealed a strong positive correlation between the number of cell infusions and treatment effectiveness:
The study noted that patients with combined severe brain injuries were less responsive to this treatment. The “non-responder” group primarily consisted of patients with the most severe forms of CP complicated by other brain damage and those who received only two or fewer UCB cell infusions.
Join families finding hope with R3 Stem Cell’s CP treatments. Call (844) GET-STEM to start your journey to improved function.
R3 Stem Cell has evolved its approach to stem cell therapy, moving away from autologous treatments to focus on more effective methods. Here’s why:
Age-related factors: As we age, so do our stem cells. This aging process affects their:
Impaired therapeutic potential: Older patients’ stem cells face challenges such as:

Umbilical cord stem cells offer significant benefits:
By focusing on umbilical cord stem cells, R3 Stem Cell ensures:
Stem cells and exosomes act in the body through several mechanisms. They do NOT become part of a patient’s DNA, which means they do not engraft into the person’s existing cells. The predominant method of action is thought to be through paracrine mechanisms, which means “cell to cell” interaction.
Unlock the potential of umbilical cord stem cells for CP. Contact R3 Stem Cell at (844) GET-STEM to learn more.
Stem cells can release a variety of molecules into the extracellular environment. These molecules, including extracellular vesicles, lipids, free nucleic acids, and soluble proteins, play crucial roles in repairing damaged tissue.
R3 Stem Cell includes stem cell exosomes in their treatment for Cerebral Palsy. These exosomes are a type of extracellular vesicle that participate in extensive cell-to-cell communication for new blood flow creation.

Experience top-quality stem cell treatments for CP. Contact R3 Stem Cell at (844) GET-STEM to learn about our rigorous standards.

R3 Stem Cell’s regenerative biologics come from umbilical cord tissue donated after scheduled c-sections. No harm comes to the baby or mother during this process. The umbilical cord tissue, normally discarded, is sent to the lab if the mother passes rigorous screening tests mandated by the USA FDA.
The lab carefully processes the umbilical cord to generate large amounts of high-quality stem cells and exosomes. The lab team, consisting of multiple PhDs, works in ISO Certified, cGMP compliant clean rooms to ensure quality that exceeds USA FDA standards.
Millions of dollars have been invested in the pharmaceutical-grade production of these biologics. The process involves:
R3 Stem Cell’s Centers of Excellence globally incorporate umbilical cord stem cell derived exosomes with umbilical cord stem cells to provide enhanced results for patients with Cerebral Palsy.
Exosomes are lipid-bound vesicles (acellular) produced by cells, containing a plethora of growth factors, cytokines, mRNA, and other proteins. They play a crucial role in:
Exosomes act as shuttles, sending nucleic acids and proteins to other cells, regulating intracellular information. They could be the mediators of many stem cell-associated therapeutic activities.
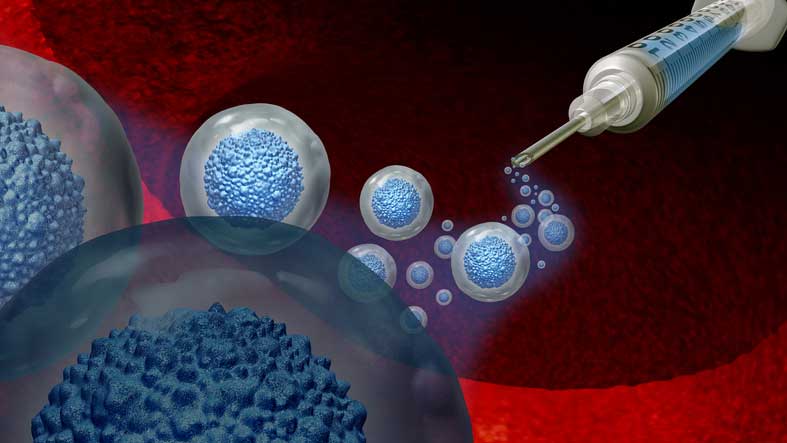
At R3 Stem Cell, we’ve observed exosomes to be “faster acting” than stem cells. We frequently use them in conjunction with stem cells to provide a “1-2 punch” for patient outcomes.
After a decade of performing over 25,000 stem cell procedures worldwide, R3 can confidently state that our regenerative procedures are safe. Our quality control during stem cell production is second to none.
Discover stem cell-derived exosomes for CP. Call R3 Stem Cell at (844) GET-STEM to learn about our advanced therapies.
Side effects are usually mild to moderate and temporary, including:
All R3 Centers are equipped to quickly resolve any allergic reactions.
Choose safe CP treatments with R3 Stem Cell. Call (844) GET-STEM to discuss our proven safety record.
Every day, R3 Stem Cell Treatment Center receives inquiries worldwide from individuals asking if stem cell therapy can help with Cerebral Palsy. Spoiler alert: It can help a lot! In this guide, we’ll go through the basics of how stem cells and exosomes work, the latest research, and what to expect with a regenerative procedure.
No. Mesenchymal Stem Cells (MSCs) are “immunologically privileged” due to their unique properties:
No. Current research concludes that MSCs and exosomes used in treatment have no tumor-forming potential. In fact, they have shown anti-tumor properties.

Our successful treatment protocol includes:
This combination ensures that millions of stem cells easily reach the brain. We use one to four million stem cells per kilogram of body weight, along with 50 to 100 billion exosomes per procedure.
R3 Stem Cell’s outcomes for Cerebral Palsy have been exceptional, with an 85% patient/family satisfaction rate year over year. Patients typically experience:
Note: Results may vary between individuals and cannot be guaranteed.
We understand the importance of making stem cell therapy affordable, as it’s not typically a permanent cure. Many patients seek additional treatments every 12 to 18 months for maintenance or further improvements.
R3 Stem Cell’s fees are less than half of what comparable reputable regenerative clinics charge.
Caution: Be wary of clinics trying to pass off Platelet-Rich Plasma (PRP) as stem cell therapy. If they only mention taking your blood for treatment, it’s NOT stem cell therapy!
Over the past ten years, R3 Stem Cell’s Centers have:
We combine safety, effectiveness, and affordability in our therapies. Internationally, we use the Intellicell, which cultures the most active MSCs to create the “smartest” stem cell in the world!
Ready to explore stem cell therapy for Cerebral Palsy? R3 Stem Cell offers FREE consultations to discuss whether regenerative therapy is right for you.
Call us today at +1 (844) GET-STEM to schedule your FREE consultation!
Don’t let Cerebral Palsy hold you back. Discover the potential of stem cell therapy with R3 Stem Cell – where advanced science meets compassionate care.
References
Disclaimer: This guide’s education does not constitute medical advice. The USA FDA considers stem cell therapy experimental. Any claims made in the Guide refer to procedures performed outside the USA.
Brand Ambassador Gallery

Free Webinar with R3 Stem Cell
All You Need to Know About Stem Cell Therapy Presented by CEO David Greene, MD, PhD, MBA!
Register Now For Free Webinar: Avoid Surgery With Stem Cell Therapy

R3 Stem Cell Master Class
Learn everything you need to know about the ever expanding field of regenerative medicine in this 8 part series that includes over four hours of entertaining content!

R3 Stem Cell International
R3 Stem Cell International includes 80 clinics in 8 countries. These Centers of Excellence treat all types of conditions with safe, effective protocols by expert stem cell physicians.
Free Stem Cell Consultation
R3 Stem Cell offers a no cost consultation to see if you or a loved one is a candidate for regenerative cell therapies including cytokines, growth factors, exosomes, and stem cells.
Provider
Partnership
The R3 Partnership Program offers providers an all-in-one regenerative practice program including marketing, consultations and booked procedures!
Success Stories
The USA Stem Cell Leader Offers Procedures In 8 Countries Including

United States

Mexico

Philippines

South Africa

Pakistan

India

Turkey

UAE
Call to schedule your free consultation at over 80 centers world!

About Us
R3 Stem Cell is a global leader in regenerative medicine, offering advanced stem cell and exosome therapies through Centers of Excellence in over 8 locations across 80 countries. Our mission is to provide first-rate, customized… regenerative
Contact Us
Main menu
Subscribe Newsletter
Contact Detail
CALIFORNIA
FLORIDA
GEORGIA
HAWAII
IDAHO
ILLINOIS
INDIANA
IOWA
KANSAS
KENTUCKY
LOUISIANA
MARYLAND
MASSACHUSETTS
MICHIGAN
MINNESOTA
MISSISSIPPI
MISSOURI
NEBRASKA
NEW JERSEY
NEW YORK
NEW MEXICO
NEVADA
NORTH CAROLINA
OHIO
OKLAHOMA
OREGON
PENNSYLVANIA
RHODE ISLAND
SOUTH CAROLINA
SOUTH DAKOTA
TENNESSEE
Disclaimer
Stem cell therapy is considered experimental and is regulated by the U.S. Food and Drug Administration (FDA), but it is not FDA-approved. R3 Stem Cell does not offer stem cell therapy as a cure for any medical condition. No statements made on this site have been evaluated or approved by the FDA. This site does not provide medical advice. All content is for informational purposes only and is not a substitute for professional medical consultation, diagnosis, or treatment. Reliance on any information provided by R3 Stem Cell, its employees, others appearing on this website at the invitation of R3 Stem Cell, or other visitors to the website is solely at your own risk. R3 Stem Cell does not recommend or endorse any specific tests, products, procedures, opinions, or other information that may be mentioned on this website. R3 Stem Cell is not responsible for the outcome of your procedure. The FDA considers stem cell therapy experimental at this point.
Copyright © 2017-2026 R3 Stem Cell. All Rights Reserved.