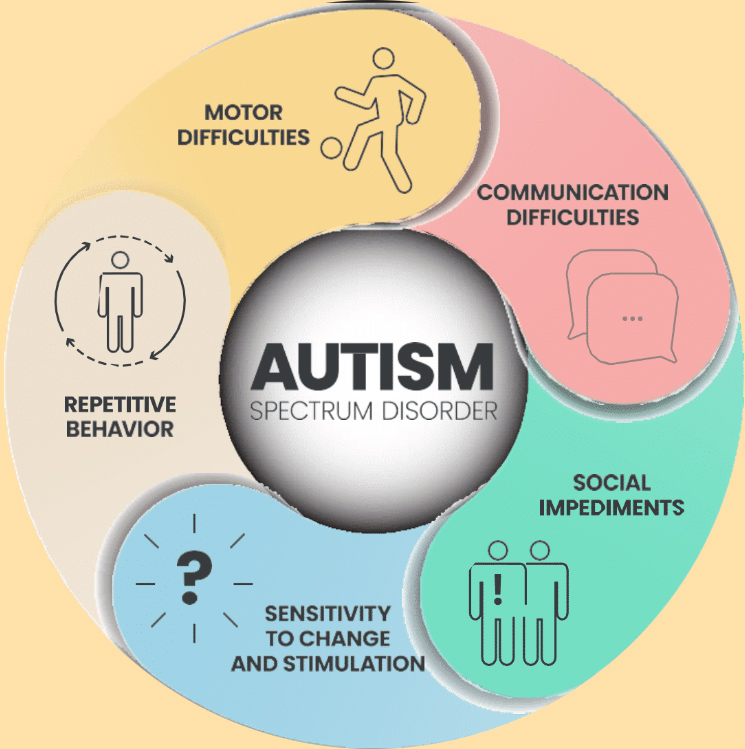
Secondly, effectiveness is critical. One of the main reasons R3’s Centers have become so popular for autism stem cell therapy is the high success rates. Over 85% of families are exceptionally happy with their child’s results. The results seen span the categories of behavior and communication. Whether it’s verbal or non- verbal improvements, the results are typically obvious.
A lot of children with ASD are hyperactive and/or aggressive with their family or friends. In line with the results Duke University saw, R3’s patients typically see a significant reduction in these behaviors.
And third, affordability is key. Because stem cell therapy for Autism is not acure, it’s important to make it affordable. Repeat therapies can help gain additional improvements for ASD children. So a lot of families seek additional treatments at R3 Stem Cell every six to eighteen months. It’s not mandatory, but something we see a lot.
Unfortunately, stem cell clinics in Colombia, China and Panama charge over $20,000 USD for treating autism with stem cell therapy. Because the one treatment cost so much, how are families supposed to budget for that every year?? R3 Stem Cell’s fees are less than half that for 100 million high quality stem cells!
When it comes to stem cell and exosome therapy for autism, R3’s protocol is safe and effective. The anesthesiologists at R3’s Centers are experts in the intrathecal application. It’s basically a “reverse” spinal tap procedure and allows millions of stem cells to safely be administered into the central nervous system.
Conscious sedation may be administered, which allows the child to be awake but not aware of the procedure. It is not general anesthesia, it’s simply a fast acting medication like Versed.
R3 Stem Cell administers a multivitamin infusion along with the stem cells, which helps to activate the cells for best results. The umbilical cord stem cells are administered IV as well as intrathecal.
R3’s experienced providers use clinical judgment and best practice protocols to decide on the amount of stem cells necessary for best results. This may include from 3 to 6 million stem cells per kilogram, and from 30 billion to 180 billion exosomes as well.
Treatments may occur on one day, or be separated into two days for patient safety. A family member is typically allowed in the room. R3’s procedure rooms have intensive monitoring equipment.