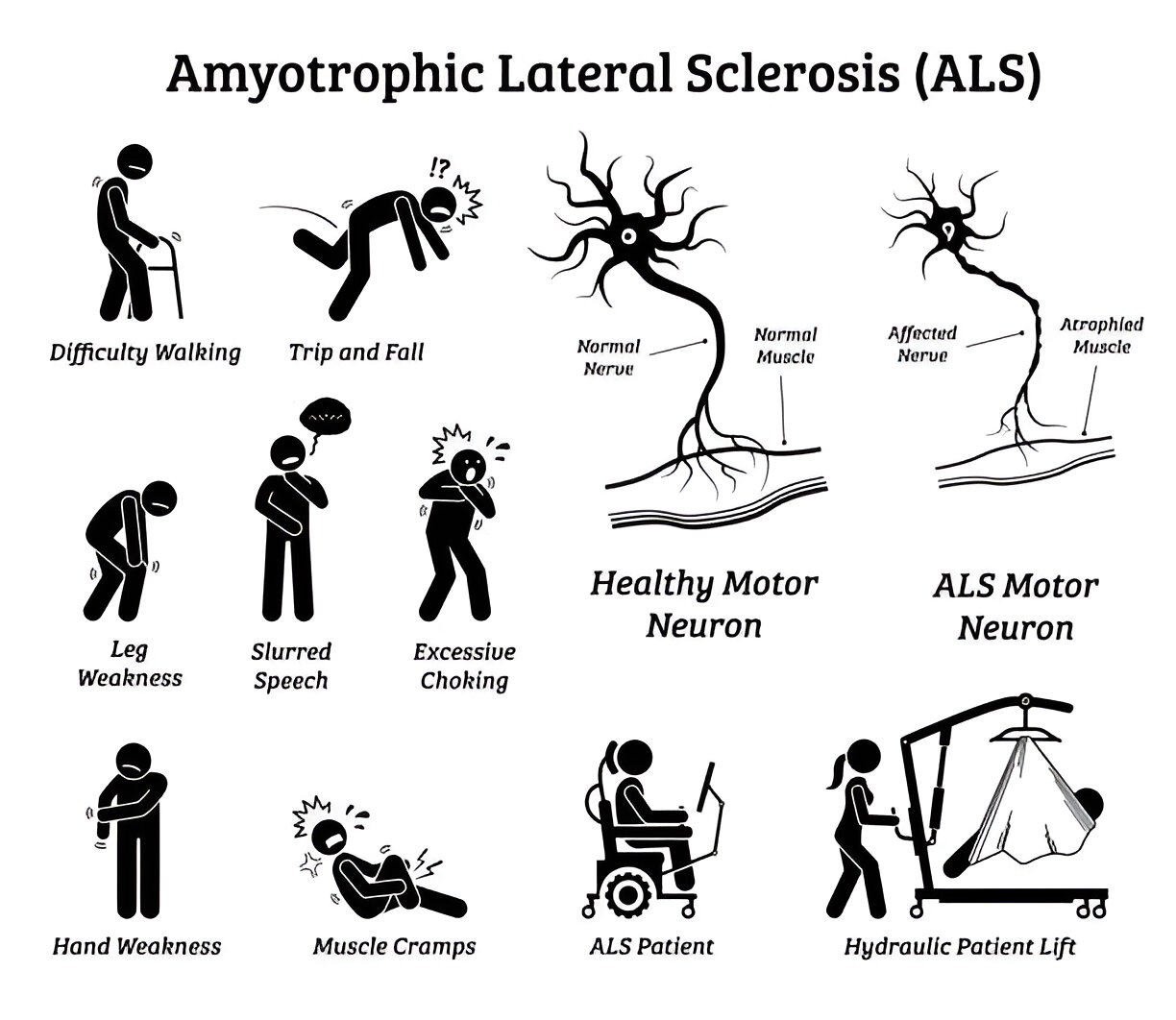
For the past decade, R3 has been successfully treating Amyotrophic Lateral Sclerosis with stem cell and exosome procedures. The procedure is performed as a combination of intravenous stem cells and exosomes along with intrathecal application.
R3’s providers use approximately two to three million stem cells per kilogram and 100 billion exosomes for the procedure.
Similar to the research mentioned above, R3’s ALS stem cell treatment outcomes have been very good! The patient satisfaction rate for ALS is 75%, with patients typically experiencing stoppage of the disease progression for a period of time. Keep in mind results cannot be guaranteed and will vary between individuals.
It may take a few months to see improvements, as it can take that long to build up new blood flow. It should be noted, again, that stem cell therapy is not a cure for ALS, and will need to be repeated every 6-12 months or so for continued benefit.
Because stem cell therapy for ALS is not a permanent cure, it’s important to make it affordable. Repeat therapies can help maintenance and/or achieve additional improvements for ALS. So a lot of patients seek additional treatments at R3 Stem Cell every six to twelve months.
R3 Stem Cell’s fees are less than half what comparable (and reputable) regenerative clinics charge.
For the past decade, R3 Stem Cell’s Centers globally have performed over 24,000 regenerative procedures in six countries. Several hundred have been for ALS
R3 combines safety, effectiveness and affordability for the therapies. Internationally, the Intellicell is used, which is culturing the most active mesenchymal stem cells to create the “smartest” stem cell in the world!