Umbilical Cord Blood Stem Cell Procedures
in Atlanta
Umbilical Cord Tissue offers exceptional regenerative capabilities at our regenerative center in Atlanta and surrounding areas.
Over the past ten years, the products of conception have become very popular for clinical use in the USA. They consist of: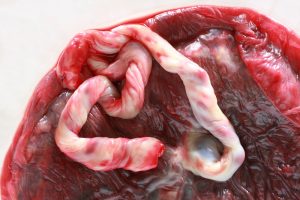
- Umbilical Cord Tissue
- Umbilical Cord Blood
- Placenta
- Amniotic Fluid
- Wharton’s Jelly
A lot of people think these tissues are a new concept to be used clinically, however, in Europe and other parts of the world they’ve been used for decades. As usual, the US is behind the curve!
Thankfully, the FDA closely regulates these biologics including who can donate, how the donation occurs, how the tissue is transported, processed, stored, and who they can be used on. According to the FDA regulations for biologics, which are called Part 1271 under Code of Federal Regulations 21, a woman under the age of 35 may be a donor. The woman is consented and also heavily screened for diseases and risk factors.
The donation occurs after a scheduled c-section, where the baby is fine. Normally, the products of conception are discarded. In these cases, however, the tissue is sterile acquired and taken right to the FDA certified lab for processing. R3 Stem Cell only works with FDA Certified labs who are also AATB Certified and have an impeccable safety record.
During processing, the labs R3 works with do NOT radiate the tissue, and only a very small amount of buffer or preservative is used. This correlates to viable cell counts that are exceptionally high (over 10 million per cc), with cell types including cytokines, growth factors, exosomes, secretomes, mRNA and stem cells.
One of the main reasons that umbilical and amniotic tissue has become so popular is that the patient’s body does not reject it. All DNA factors are removed during processing, so it becomes immunologically privileged. No blood typing is necessary!
Another reason for it’s popularity is that the cell counts and types are WAY higher than one’s own Bone Marrow or Adipose could ever hope to yield. Every year that goes by in a person’s life represents another large drop in the body’s ability to produce stem cells and other regenerative cells. Coupled with the potential for complications, it just doesn’t make sense to have one’s own cells used when such a safe, effective alternative is available.
When the regenerative biologics are injected into the body, such as the knee, an immense reaction occurs in the body. A lot of what happens involves signaling between the injected biologic and one’s own regenerative cells. The work begins with a complex interaction that includes stem cells differentiating into specialized cells needed in the area (e.g. cartilage, tendon), growth factors directing the construction, cytokines combating inflammation, and additional functions by the exosomes and secretomes.
Who Can Benefit from the Regenerative Procedures?
 There are now hundreds of peer reviewed papers describing how well the amniotic and umbilical tissue works for musculoskeletal conditions such as arthritis, tendonitis, sports injuries, plantar fasciitis and wound care.
There are now hundreds of peer reviewed papers describing how well the amniotic and umbilical tissue works for musculoskeletal conditions such as arthritis, tendonitis, sports injuries, plantar fasciitis and wound care.
It’s truly been amazing to see the outcomes being experienced at R3 Centers, with hundreds of testimonials pouring in annually. Patients upwards of 100 years old have been treated successfully for degenerative arthritis. Most patients avoid the need for joint replacement, and return to activities that have not been possible for quite a while.
Outside of the musculoskeletal realm, excellent outcomes are being seen for organ failure, autoimmune disease, neurodegenerative conditions, neuropathy, post-stroke, dementia, erectile dysfunction, hair loss, and a plethora of other conditions. Because they are so safe, physicians have been able to try the treatments on a lot of conditions. Intersstingly, one way treatments have been shown to be effective for a condition is when they are used for something else and as a collateral benefit a different condition gets better!
Are they Safe?
They are VERY safe. In over 10,000 cases, we have only seen a couple superficial infections that resolved with antibiotics. No rejection, disease transmission, allergic reaction etc. Having said that, there is always the possibility of a complication occurring just as with any injection or infusion.
How well do they work and for how long?
This is variable for two reasons:
- People are very different when it comes to their physiology and how they react to the biologics. Some people will have relief for many years, others much shorter than that. The overall effectiveness we have seen averages 85%.
- The second reason is severity of one’s condition. There is a big difference between Stage 2 and Stage 4 kidney disease as an example. Repetitive treatments may be necessary for an end stage situation.
Will they work well for a bone on bone situation?
Surprisingly, the answer is yes. When R3 first began we used to recommend providers not to work on bone on bone situations. We were wrong, as providers notified us that the outcomes were great. It doesn’t take much new cartilage formation to provide sufficient padding to obtain great relief.
How does the FDA regulate the biologics and are they covered by insurance?
The FDA strictly regulates amniotic and umbilical tissue as a Biologic (not a drug). The labs are FDA Certified, but the biologic does not go through a clinical trial like a drug would. Therefore, the biologics are neither FDA approved or denied, simply regulated.
As with all new technology in the US, it will take a number of years for insurance companies to start covering them. It will probably be another seven years or so before that happens.
What’s the bottom line?
There is a reason that over 95% of people who call R3 or come in for an appointment are considering treatment with amniotic and umbilical cord tissue. It’s because the biologics are safe, effective, and readily available.
R3 has been doing this for a long time with Centers of Excellence. It is critical to keep in mind that all of the regenerative biologics are NOT created equally at the lab. Our labs are diligent and careful about the processing to ensure the highest cell viability possible. We have third party assays to show the difference, and our outcomes with patients are second to none.
R3’s Center of Excellence in Atlanta offers a free consultation. Call today to set up yours!